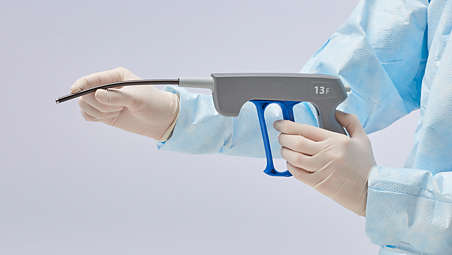
Specifications
- Model number 560-009
-
Model number 560-009 Size - 9F
Device inner diameter - 9.1F / 0.119" / 3.0 mm
Device outer diameter - 14.4F / 0.187" / 4.8 mm
Outer sheath outer diameter - 18.9F / 0.245" / 6.3 mm
Working length - 6.1" / 15.5 cm
-
- Model number 560-011
-
Model number 560-011 Size - 11F
Device inner diameter - 11.1F / 0.145" / 3.6 mm
Device outer diameter - 16.4F / 0.213" / 5.5 mm
Outer sheath outer diameter - 20.9F / 0.271" / 6.9 mm
Working length - 6.1" / 15.5 cm
-
- Model number 560-013
-
Model number 560-013 Size - 13F
Device inner diameter - 13.1F / 0.171" / 4.3 mm
Device outer diameter - 18.4F / 0.239" / 6.1 mm
Outer sheath outer diameter - 22.9F / 0.297" / 7.6 mm
Working length - 6.1" / 15.5 cm
-
- Model number 560-009
-
Model number 560-009 Size - 9F
Device inner diameter - 9.1F / 0.119" / 3.0 mm
-
- Model number 560-011
-
Model number 560-011 Size - 11F
Device inner diameter - 11.1F / 0.145" / 3.6 mm
-
- Model number 560-009
-
Model number 560-009 Size - 9F
Device inner diameter - 9.1F / 0.119" / 3.0 mm
Device outer diameter - 14.4F / 0.187" / 4.8 mm
Outer sheath outer diameter - 18.9F / 0.245" / 6.3 mm
Working length - 6.1" / 15.5 cm
-
- Model number 560-011
-
Model number 560-011 Size - 11F
Device inner diameter - 11.1F / 0.145" / 3.6 mm
Device outer diameter - 16.4F / 0.213" / 5.5 mm
Outer sheath outer diameter - 20.9F / 0.271" / 6.9 mm
Working length - 6.1" / 15.5 cm
-
- Model number 560-013
-
Model number 560-013 Size - 13F
Device inner diameter - 13.1F / 0.171" / 4.3 mm
Device outer diameter - 18.4F / 0.239" / 6.1 mm
Outer sheath outer diameter - 22.9F / 0.297" / 7.6 mm
Working length - 6.1" / 15.5 cm
-
- Product availability is subject to country regulatory clearance. Please contact your local sales representative to check the availability in your country.
- TightRail is distributed by LifeSystems in Australia.
- Always read the label and follow the directions for use.
- Philips medical devices should only be used by physicians and teams trained in interventional techniques, including training in the use of this device.
- Philips reserves the right to change product specifications without prior notification.
- ©2025 Koniklijke Philips N.V. All rights reserved. Trademarks are the property of Koninklijke Philips N.V. or their respective owners.